A Breakthrough In Non-Invasive Treatment for Chronic Low Back Pain and Osteoarthritis: Insights from a Recent Study
by Dr. Francisco Cidral, ND, PhD
Low back pain (LBP) is the most prevalent musculoskeletal condition globally and the leading cause of disability worldwide. As
of 2020, an estimated 619 million people suffered from LBP, with projections suggesting this number could rise to 843 million by 2050 (GBD, 2021). Associated to LBP, lumbar spine osteoarthritis (OA) is particularly common, with prevalence estimates ranging from 40% to 85% (Goode et al., 2013). Such debilitating conditions, often diminish the quality of life and hinder daily activities. Traditional treatment approaches for OA, including pain management through medication, physical therapy, and in severe cases, surgical intervention, offer limited relief and are often accompanied by undesirable side effects (Corp et al., 2021; Urits et al., 219). However, recent advancements in regenerative medicine present a promising alternative: non-invasive topical treatments derived from stem cells.
Regenerative Medicine: A New Horizon
Regenerative medicine, particularly stem cell therapy, has been hailed as a revolutionary approach to treating a variety of conditions, including osteoarthritis (Miranda et al., 2023). Stem cells, especially those derived from sources such as Wharton’s jelly
and the amniotic membrane, possess powerful anti-inflamma- tory properties and the ability to promote tissue regeneration. Traditionally, these stem cells have been applied via invasive procedures, such as autologous stem cell transplantation, which, despite their potential, come with significant challenges. The invasiveness of these procedures, coupled with the difficulty in selecting suitable candidates for intervertebral disc injections, has limited their widespread adoption (Ansari et al., 2018; Main et al., 2021; Zand and Lai, 2020).
The recent focus on bioactive molecules, growth factors, cytokines, and telomerase derived from mesenchymal stem cells (MSCs) offers a promising non-invasive alternative. These components, harvested from MSCs, may provide therapeutic benefits without the need for invasive procedures (Roszkowski, 2024; Al-Azab et al., 2022; Panchalingam et al., 2015). This has led to the development of innovative products like Rheegen Alpha and Rheegen Omega, topical transdermal creams infused with stem cell-derived biomolecules, designed to alleviate symptoms associated with lumbar OA.
The Study: Evaluating the Efficacy of Stem Cell creams
To explore the potential of these topical treatments, a groundbreaking study was conducted, enrolling eighty participants aged over 40 years, all clinically diagnosed with lumbar OA. The study spanned four weeks, during which participants applied Rheegen Alpha cream to their lumbar region before activity or exercise, and Rheegen Omega after activity or showering.
Pain levels, the primary metric for evaluating the treatment’s efficacy, were assessed using the Visual Analogue Scale (VAS) at the beginning of the study and after the treatment period. The study’s statistical analysis, performed using GraphPad Prism version 8.0, revealed a significant reduction in pain levels (p < 0.0001) with a large effect size (Cohen’s d = 2.25). The results, showed a 51% decrease in reported pain levels, indicating the creams’ substantial impact on reducing pain.
A Safe and Effective Treatment
One of the most compelling findings from the study was the absence of any adverse allergic reactions among participants, underscoring the safety of these topical treatments. The significant reduction in pain, combined with the safety profile, positions these stem cell creams as a potentially transformative treatment for individuals suffering from lumbar OA.
The promising results are believed to be linked to the bioactive biomolecules in the creams, which are derived from human Wharton’s jelly mesenchymal stem cells (WJMSCs).
These biomolecules may support cell differentiation and tissue regeneration, offering a non-invasive solution to a condition that traditionally required more invasive approaches.
Conclusion
The development of topical stem cell creams represents a significant step forward in the treatment of lumbar osteoarthritis. With their non-invasive application, these creams offer a safe and effective alternative to traditional treatments, providing substantial relief from pain without the risks associated with more invasive procedures. As research continues, there is hope that these advancements in regenerative medicine will not only improve the quality of life for those suffering from chronic low back pain but also transform the broader landscape of osteoarthritis treatment.
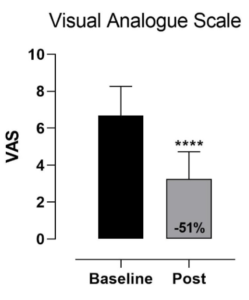
Figure 1: Visual Analogue Scale scores were significantly reduced in patients with lumbar osteoarthritis after a four-week application of Rheegen Stem Cell Creams. Each data point represents the average of 80 patients. Data did not pass the Shapiro-Wilk normality test and a Wilcoxon matched-pairs signed rank test
was performed. Data are presented as mean ± SD. **** p < 0.0001, baseline versus post-treatment.
FRANCISCO CIDRAL, ND, MSC, PHD, POSTDOC, is the founder and CEO of Scientifica Consulting. He holds a master’s degree and PhD in neurosciences and a postdoctorate in health sciences. Cidral is a professor of integrative medicine and neurophysiology, with a specialization in laser acupuncture and photobiomodulation. He has authored more than 35 scientific publications and books. Cidral is a board member of various scientific journals and international research groups.