Understanding Brainwave Entrainment: A Deep Dive into Neuronal Activity
Neurons are the fundamental signaling units of the brain. Each neuron has a basic structure consisting of dendrites, the cell body (soma), and the axon. Dendrites receive signals from other nerve cells, the somaacts as the cell’s metabolic center housing the nucleus, and the axon is responsible for transmitting signals. An axon can carry electrical signals over distances ranging from 0.1 mm to 1 m. These electrical signals, known as action potentials, can propagate at speeds of 1 to 100 m/s. Action potentials enable the brain to receive, analyze, and transmit information (Kandel et al., 2023).
Human brainwaves are rhythmic patterns of neural activity in the central nervous system (CNS), representing the electrical voltages produced by groups of neurons activating together, typically measuring a few millionths of a volt (Abhang et al., 2016; Tatum, 2014). These waves can be characterized by frequency, amplitude, and phase, coordinating brain activity and facilitating signal transmission between brain regions (Headley & Paré, 2017). It is known that coherent and functional brainwave patterns are crucial for successful task execution, whether physical or mental (Tang et al., 2016) and that different brain regions can emit different frequencies simultaneously (Abhang et al., 2016).
Excitatory and inhibitory interactions within brain circuits operate across various timescales, influencing the emergence of oscillations at different frequencies. These oscillations span a spectrum from slowest to fastest, encompassing delta, theta, alpha, beta, gamma, and sharp-wave ripples. They display distinct temporal and regional patterns throughout the brain and are theorized to play essential roles in cognitive functions, such as memory (Adaikkan and Tsai, 2019).
Brain oscillatory rhythms in the 1-30 Hz range can be modulated or entrained by external stimuli. Entrainment occurs when a group of neurons in a stimulated area synchronizes their activity with an external rhythm (Figure 1). This process involves two main effects: first, an increase in signal strength as more neurons align their phases with the external rhythm; second, the synchronization of the entire neuronal population to this rhythm. This synchronization doesn’t happen instantly but evolves over time, influenced by the intensity of the stimulation (Thut et al., 2011; Hanslmayr et al., 2019).
Brainwaves are evident in extracellular electric fields and can be detected by various methods. The most popular is EEG, a noninvasive technique using scalp electrodes (Abhang et al., 2016; Joshi et al., 2019). More invasive methods like electrocorticography and local field potential recording provide higher spatial and temporal resolution but require electrode implantation in the cerebral cortex (Joshi et al., 2019).
Therefore, Brainwave Entrainment (BWE) therapy posits that auditory or visual stimulation at specific frequencies can modulate the brain’s electrocortical activity. This potential is intriguing because it suggests the possibility of inducing specific physiological and psychological states through designed external stimuli with particular frequency bands (Ingendoh et al., 2023).
By understanding and harnessing these rhythmic brainwave patterns, BWE therapy aims to enhance mental and physical performance, opening new avenues for therapeutic and cognitive interventions.
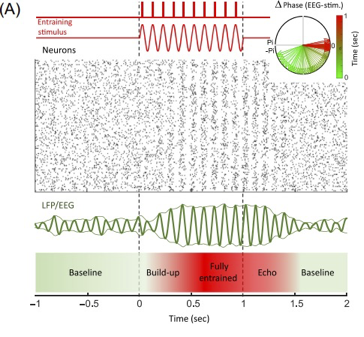
Figure 1. Neurons are entrained either by a continuous stimulus (sine wave) or pulses of stimuli. The raster plot displays simulated spiking activity in a neural population, while the local field potential (LFP)/EEG (electroencephalography) illustrates the population-level activity. The upper right panel indicates the phase difference between the entraining stimulus and population activity, with colors marking time from the beginning (green) to the end (red) of entrainment.
Source: Hanslmayr et al., 2019
References
Abhang PA, Gawali BW, Mehrotra SC. Technological Basics of EEG Recording and Operation of Apparatus. Introduction to EEG- and Speech-Based Emotion Recognition. 2016;19–50. doi: 10.1016/B978-0-12-804490-2.00002-6
Adaikkan C, Tsai LH. Gamma Entrainment: Impact on Neurocircuits, Glia, and Therapeutic Opportunities. Trends in Neurosciences. 2019; 43(1):24-41. doi:10.1016/j.tins.2019.11.001
Hanslmayr S, Axmacher N, Inman CS. Modulating Human Memory via Entrainment of Brain Oscillations. Trends Neurosci. 2019 Jul;42(7):485-499. doi: 10.1016/j.tins.2019.04.004.
Headley DB, Paré D. Common oscillatory mechanisms across multiple memory systems. NPJ Sci Learn. 2017;2:1. doi: 10.1038/s41539-016-0001-2.
Ingendoh RM, Posny ES, Heine A. Binaural beats to entrain the brain? A systematic review of the effects of binaural beat stimulation on brain oscillatory activity, and the implications for psychological research and intervention. PloS one, 2023;18(5): e0286023. doi: 10.1371/journal.pone.0286023
Joshi SR, Headley DB, Ho KC, Paré D, Nair SS. Classification of Brainwaves Using Convolutional Neural Network. Proc Eur Signal Process Conf EUSIPCO. 2019 Sep; 2019:10.23919/eusipco.2019.8902952. doi: 10.23919/eusipco.2019.8902952
Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science (6th ed.). McGraw-Hill. 2023.
Tang HY, Riegel B, McCurry SM, Vitiello MV. Open-Loop Audio-Visual Stimulation (AVS): A Useful Tool for Management of Insomnia? Appl Psychophysiol Biofeedback. 2016 Mar;41(1):39-46. doi: 10.1007/s10484-015-9308-7.
Tang HY, Vitiello MV, Perlis M, Riegel B. Open-Loop Neurofeedback Audiovisual Stimulation: A Pilot Study of Its Potential for Sleep Induction in Older Adults. Appl Psychophysiol Biofeedback. 2015 Sep;40(3):183-8. doi: 10.1007/s10484-015-9285-x
Tatum, W. Handbook of EEG interpretation (2nd ed.). New York: Demos Medical Publishing LLC, 2014.
Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol. 2011 Jul 26;21(14):1176-85. doi: 10.1016/j.cub.2011.05.049.
Timmermann DL, Lubar JF, Rasey HW, Frederick JA. Effects of 20-min audio-visual stimulation (AVS) at dominant alpha frequency and twice dominant alpha frequency on the cortical EEG. Int J Psychophysiol. 1999 Apr;32(1):55-61. PMID: 10192008.
